What is CALUX®?
The CALUX® Assay (Chemically Activated Luciferase Expression) is a screening bioassay for dioxin-like compounds (PCDDs, PCDFs, and DL-PCBs) using a recombinant cell line developed by Dr. Michael Denison, UC Davis, and patented in December 1998.
Why CALUX®?
It can measure dioxin-like compounds (PCDDs, PCDFs, and DL-PCBs) faster and cheaper than the traditional instrumental analysis method (GC/HRMS ).
GC/HRMS
CALUX®
Turnaround
Sample Amount
Throughput
How does it work?
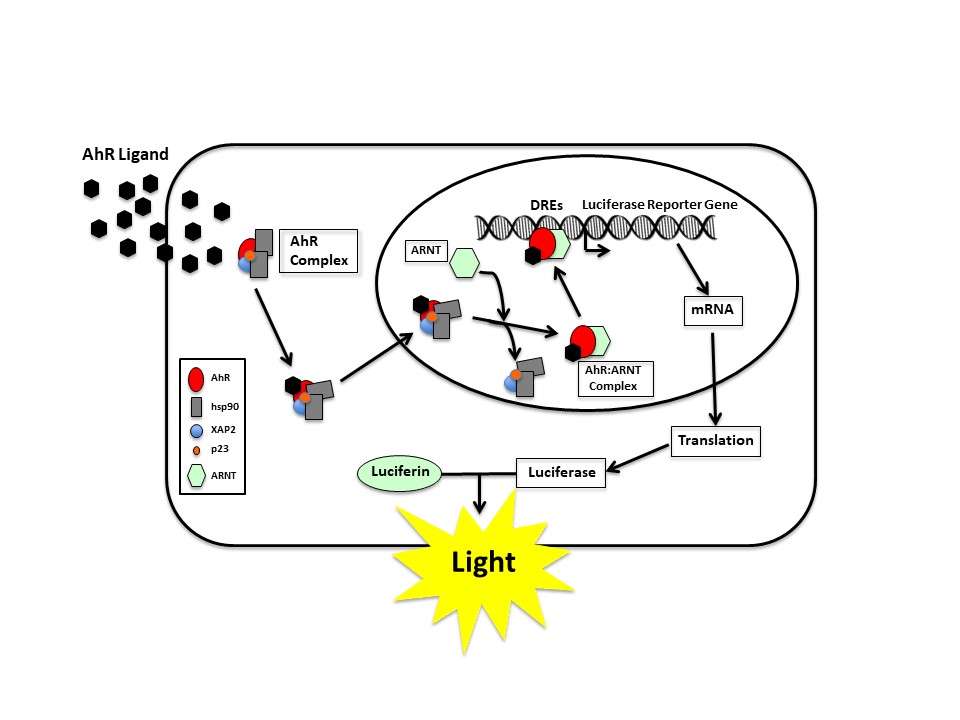
The mammalian cell was genetically engineered and involved stable insertion of an AhR-responsive luciferase reporter gene into the DNA of the cell. When ligands (dioxins and other AhR agonists) enter into the cell, the AhR signaling pathway is activated, the luciferase gene is expressed and luciferase is produced. When luciferin, the substrate for luciferase, is broken down by luciferase, light is produced and it can be easily measured. The amount of luciferase activity is directly proportional to the concentration of ligand-activated AhR and thus the amount of AhR agonist). This bioassay allowed measurement of the relative amount of AhR ligands (agonists) in a sample extract and when the extract has been subjected to a clean-up procedure that allows isolation of AhR-active dioxins and dioxin-like chemicals, the induced light output is directly related to the potential dioxin-like toxicity of the extract.
Who should use CALUX®?
The CALUX® Assay can be used for dioxin testing of environmental matrices such as soil, sediment, ash, water, exhaust gas, as well as biological matrices such as blood, breast milk, fatty tissue, and food matrices as fish, meat or dairy products. It is also been shown to be useful for screening of dioxins present in feed additives..
GC/HRMS vs. CALUX®
GC/HRMS:
- Provides data on individual chemical congeners
- Congener data can be corrected by TEF values to estimate TEQ or “toxicological risk”
CALUX®:
- Rapid identification of significant levels of dioxins and dioxin-like chemicals
- Provides a direct estimate of TEQ or “toxicological risk”
- Can be used in a high throughput screening format
- Cost effective quantification of TEQ for Dioxins
Learn More
- CALUX® is validated as method 4435 by the U.S. EPA
- CALUX® is certified by the EU (Commission Directive 2002/69/EC), and government organizations in Japan (JIS0463), Belgium (SIPH, Federal Feedings Laboratory), Poland (National Veterinary Research Institute) and Taiwan (NIEA S901.60B).
- It can also be used for monitoring and screening dioxin inventories, generation control and remediation. (case: superfund, worldbank)
- CALUX® can be measured as low as 0.1-1pg TEQ/g of dioxin conjenars.
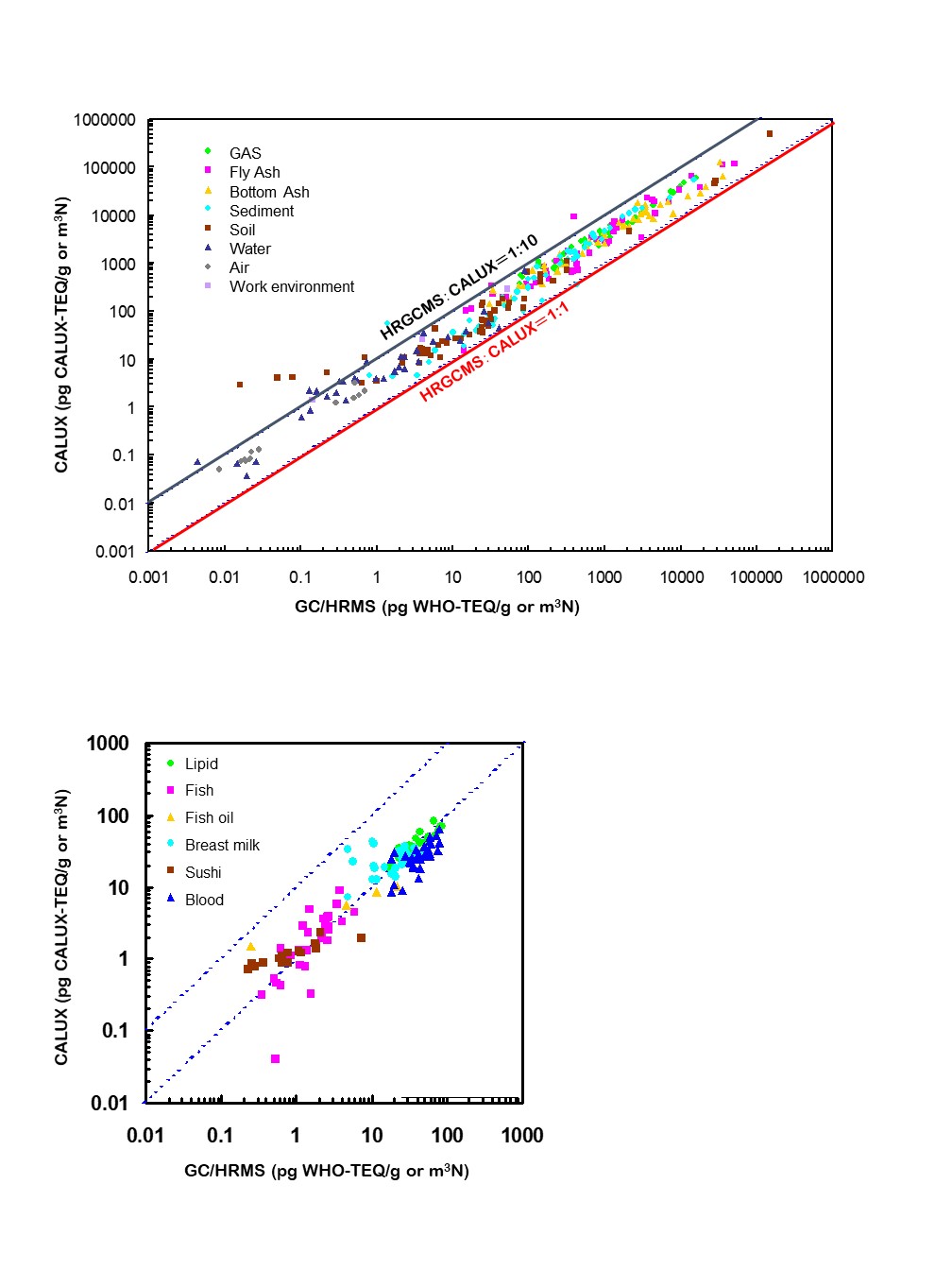
ALUX results from many different matrices have high correlations with TEQ estimates from GC/HRMS analyses.
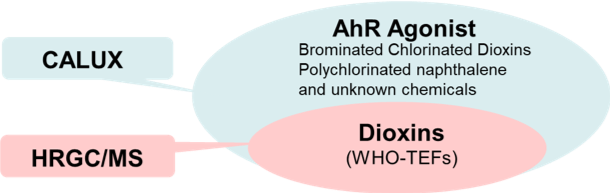
CALUX® for AhR-active chemicals
AhR Agonists (Contaminants of Emerging Concern (CECs)
When combined with an appropriate extraction and cleanup method, the CALUX® bioassay provides a targeted approach for screening and not only allows detect not only detection of toxic chlorinated dioxins and dioxin-like compounds (PCDDs, PCDFs, and DLdl-PCBs), but also brominated and mixed bromo/chloro-dioxins and dioxin-like chemicals and other halogenated aromatic hydrocarbons (ll AhR Agonist (Brominated Chlorinated Dioxins, pPolychlorinated naphthalenes, polychlorinated/brominated parabens and others unknown chemicals). However, since the AhR signaling pathway can be activated by a wide variety of structurally diverse chemicals, CALUX® analysis of crude sample extracts provides a novel avenue for screening for the presence of a much broader range of AhR active chemicals.
Due to diminishing of new water sources, water reclamation, recycling, and reuse are becoming major issues. Maintaining a water quality that is protective of both human health and the environment is of paramount importance. Meanwhile the presence of chemicals in municipal wastewater (the “source” of recycled water) is well known and should be attenuated before potable reuse and discharge into the environment. The chemical universe is evolving at a rate that is extremely challenging for traditional risk assessment paradigms, and the application of bioassay approaches to help facilitate quality monitoring is now being considered in many countries for comprehensive evaluation of constituents of emerging concern (CECs).
In December 2018, the California State Water Board amended its Recycled Water Policy, to require cell bioassay-based water monitoring using AhR and estrogen receptor cell bioassays starting in April of 2020.
For Estrogen Receptor-α (ERα)
What is LUMI-CELL®?
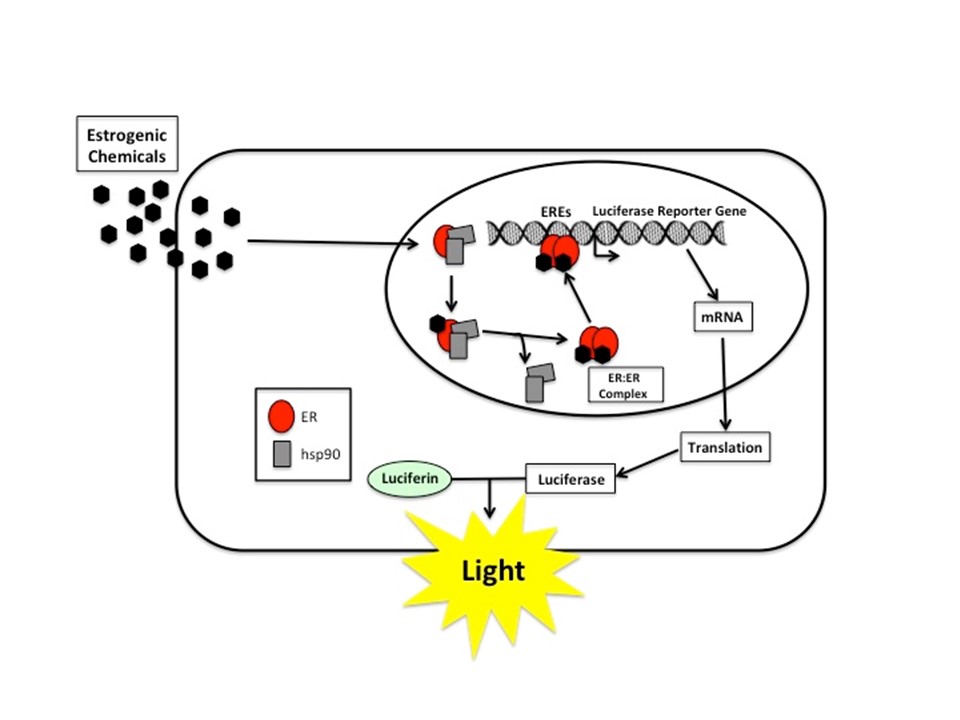
Similar to the CALUX® bioassay, LUMI-CELL® is a screening assay using a recombinant cell line (Human breast Carcinoma cell “VM7Luc4E2”) containing a stably integrated ER-responsive luciferase reporter gene for detection and relative quantitation of estrogenic or antiestrogenic activity of chemicals or extracts. The LUMI-CELL® bioassay was endocrine disruptor compounds developed by Dr. Michael S. Denison, UC Davis .
Why LUMI-CELL®?
Traditional instrumental analysis methods can provide outstanding high-resolution quantitative results for the known estrogenic chemicals that are being measured. However, the major limitations of instrumental analysis methods for estrogenic chemical screening and monitoring purposes is that they are not able detect unknown estrogenic chemicals and they can’t provide a measure of the total estrogenic activity of a sample extract. In contrast, ER-based cell bioassays provide an avenue in which to detect all estrogenic chemicals in a sample extract (known and unknown) and determine its total estrogenic activity. These aspects, combined with the fact that cell bioassays are also significantly less time-consuming and costly as instrumental analysis make them the preferred method of choice for open screening and monitoring purposes.
Who should use LUMI-CELL®?
The LUMI-CELL® bioassay has been shown to be and effective method for measuring the estrogenic activity of many matrices, including: food, feed stuffs, biologicals, sediments, air, water, pharmaceuticals, cosmetics, pesticides, steroid products and many more. This bioassay is also able to detect total estrogenic or antiestrogenic activity for a sample, with detection limits of ~0.04pg/g.
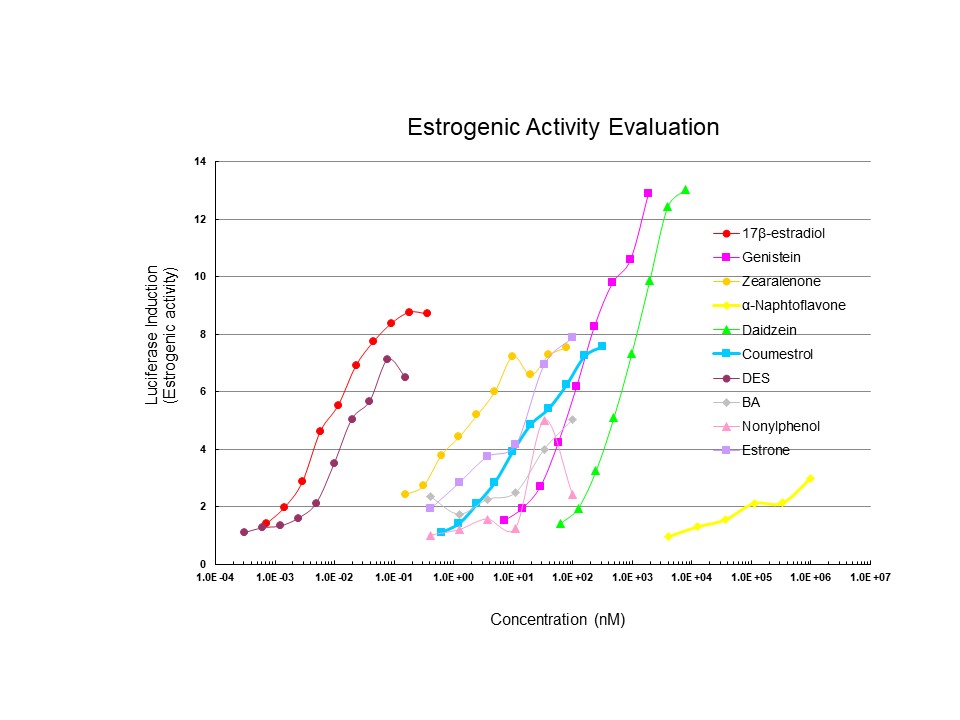
Example
This figure shows the results of analysis of the estrogenic activity of selected chemicals. While the estrogenic potency and efficacy of the synthetic estrogen, Diethylstilbestrol (DES), was similar to that of 17β-estradiol (E2), numerous phyoestrogens (Genistein (from soy), Daidzein, and Zearalenone (mycotoxin)) and other compounds showed reduced potency but were efficacious ER activators. These results demonstrate the utility of the LUMI-CELL® bioassays for detection and relative quantitation of estrogenic chemicals.